This is an analogy of cells using animal cells. You probably may not have heard of some of the organelles.
Body: Earth
System: Continent
Organ: Country
Tissue: State
Cell: City
Cell membrane: City border with guards to monitor people entering and leaving the city
Free Ribosome: Individual shop
Ribosome on Rough Endoplasmic Reticulum (RER): Shop part of a franchise
RER: Franchise Headquarters
Smooth Endoplasmic Reticulum (SER): Recycling Plant
Vesicle pinched from RER: Transport vans for shop products
Vesicle pinched from SER: Transport vans for recycled products
Golgi Apparatus: Factory for processing, refining and modifying imported products
Vesicle fusing with Golgi Apparatus: Transport vans arriving and unloading goods
Vesicle pinching off from Golgi Apparatus: Transport vans with ready-to-go goods
Vesicle fusing with cell membrane: Transport vans unloading goods for export out of city
Cytoplasm: Free land
Nucleus: Government
Chromatins: High post officials
Centriole: Civil servants
Nucleolus: President/Prime Minister
Vacuole: Storage Plant
Mitochondria: Power Plant
If you happen to find that my analogy is incorrect at some parts, please let me know so I can change it. Thanks!
Wednesday, July 1, 2009
Cells: An Analogy
Thursday, April 30, 2009
Crystallisation
Tuesday, April 21, 2009
Separating Mixtures
1) A mixture of non-magnetic materials and iron
Method: Use a magnet
2)A mixture of water, a solid that can dissolve in water and a solid that cannot (eg. water, salt and sand)
Method: filter the mixture to remove the sand, then use distillation to separate the salt and the water
3) 2 liquids that do not mix (eg. water and oil)
Method: Slightly more complicated. There is a special machine built to do so. It is called the API oil-water separator. You can find out about it in Wikipedia at this site: http://en.wikipedia.org/wiki/API_oil-water_separator
4) Air and petroleum
This is definitely more difficult... There is a special type of machine that is used. It is big, bulky and complicated. You can see it at these sites:
Saturday, April 18, 2009
The aftermath of coke and mentos
Group members: Sarah Tay, Rachel Seah, Stella (who, by the way, took all the photos, thanks!) and me
Objective: To find out how the number of Mentos affects the height of the fountain.
Hypothesis: The more mentos put in, the higher the fountain will go.
Items used: Several tubes of mentos, three bottles of 1.5 litre coke, two meter rulers, two measuring cylinders, one of which got jammed, and one poncho that made me look like Little Red Riding Hood.
Finally, we put in 10 mentos at once. Of course, being me, I put the stupid ruler crooked so the reading was inaccurate.
Our final conclusion was that we were correct, but up to a certain point the coke would stop dissolving the mentos and we would need to add more coke instead.







Tuesday, April 7, 2009
Separation of mixtures
1. Hand Separation
Example: Separating salt and sand
2. Filtration
Example: Sand and Water
3. Distillation
Example: Alcohol and Water
4. Chromatography, for separating different substances to determine whether they are banned substances
5. Etc. There are a whole lot of other techniques but they are too complicated and not required at this level.
http://www.chemteam.info/Matter/SeparationOfMixtures.html
Friday, April 3, 2009
The Elements

Also, for those who enjoyed Miss Liang's elements song in class today, I am posting a video, probably by the same makers of Li Shan's Newton video as both use Lego.
Bibliography
http://www.flickr.com/photos/dullhunk/2053007240/
http://en.wikipedia.org/wiki/Periodic_table_%28large_version%29
http://en.wikipedia.org/wiki/Periodic_table
Tuesday, March 31, 2009
National Vertical Marathon 2009
Links:
http://en.wikipedia.org/wiki/Republic_Plaza,_Singapore
Friday, March 27, 2009
Tallest buildings
1.OUB Centre 280 m, 63 floors
2.UOB Plaza 1, 280 m, 66 floors
3. Republic Plaza, 280 m, 66 floors
As for the world's tallest buildings, here they are!
1. Burj Dubai, Dubai, The United Arab Emirates
Built in 2009
162 floors, 818 m
2.Taipei 101, Taipei, Taiwan
Built in 2004
101 floors, 508m
3. World Financial Center, Shanghai, China
Built in 2008
101 floors, 492m
Bibliography:
http://en.wikipedia.org/wiki/List_of_tallest_buildings_in_Singapore
http://www.infoplease.com/ipa/A0001338.html
Thursday, March 26, 2009
Volcanic eruption 5 times in a row
Lab Symbols Video
Hello, this is a funny lab symbols video from Youtube. The wording may be a bit faint though, so you might have to pause and rewind to see some parts clearly. Enjoy!
Friday, March 20, 2009
Between Physics and Chemistry
http://www.youtube.com/watch?v=kMXPOqovSBs
Basically, this is what happens:
The ingredients required to create the sensational explosion is as follows.
1. CO2 gas found in soft drinks
2. Artificial Sweetener (which is why diet coke is better than normal coke)
3. Gum Arabic
4. Gelatin
5. The process of Nucleation. Mentos surface is full of tiny craters, and during this process the aforementioned ingredients interact with each other, causing the CO2 to escape to the surface. This is evident from the fact that non-mint mentos, with a waxed surface, reacts very slowly or not at all as the wax covers the craters.
Necco wafers consist of: sugar, corn syrup, gelatin, gum, colorings and flavorings. From the ingredients, you can tell that Necco wafers are more of powdery candy than wafers as there is no flour in it. I think this would be an interesting experiment to try. However, Necco wafers are manufactured in the USA, so it would be hard to obtain them here in Singapore. Thus, to try this experiment, one could just add gum and gelatin to Pepsi as the other ingredients are redundant. These are pics of necco wafers below.
I think I will try this when I have the time an post more about it.
Links:
Tuesday, March 10, 2009
Convection currents
I think that a tall person would probably feel warmer. This is because they are higher up and the warm air will circle around them. Surprisingly, I have also observed this very often: My taller friends would be sweating profusely, while my shorter friends are huddling together trying to get warm!
As for the second question, I don't think it makes a difference since every level has air-conditioning! As for people feeling "cooler" on the top levels, it is because that is where the cooling unit for the entire building is.
Monday, March 9, 2009
Ice, Water, salt and Ice cream
Secondly, if a glass is filled with water and ice cubes are placed in the glass, when the ice cubes melt will the water level rise? The answer is no, the water level will NOT rise. At first, I also thought that the water level would rise. However, once I read the explanation, it made perfect sense. The reason is that when ice melts, its volume is still the same so it will take up the same amount of space that it took up when it had not melted yet.
Lastly, we were asked to research on the effects of impurities on water. Well, this is what I know: In cold countries, salt, an impurity, is thrown on the roads to melt the snow and ice. This is because impurities lower the melting point of ice. This same concept is applied to make ice cream. You can just pour sugar and milk into a freezer bag and seal it, then place it into a larger bag, already filled with crushed salt. Make sure both bags are tightly sealed, and then shake the bags vigorously(but not so vigorously that they split open or smash!) After some time, the contents should solidify and turn into delicious homemade ice cream!
I found a video on Youtube summarising the above instructions in a 1min 18 sec clip. Enjoy!
http://www.elmhurst.edu/~chm/vchembook/122densityice.html
http://sg.answers.yahoo.com/question/index;_ylt=AqAdCXkAnMkY8xceJjXqoAQh4wt.;_ylv=3?qid=20080208133726AAWySCd
http://www.wikihow.com/Make-Ice-Cream
Tuesday, March 3, 2009
Challenger disaster
On January 28, 1986, the Challenger was supposed to conduct the TISP (Teacher in Space Program). In other words, for the very first time, a teacher would be boarding the Challenger to visit space. However, the temperature was too cold (29 degrees F). The engineers who made the Challenger told the NASA managers that they should wait until the temperature reached 58 degrees F. This is because the low temperatures might cause the O-rings to not seal properly. The O-rings kept hot gases, preventing them from escaping.
However, NASA really wanted to launch the flight, so the managers decided to give the go-ahead anyway. Just as Challenger was pulling away from Earth, one of the O-rings failed to seal, causing the hot gas to escape. Flames shot out from one slide of the rocket and licked at the hydrogen tank. Seconds later, the tank exploded, and all seven crew members, including the teacher, died on the spot.
This shows how important it is to consider the safety facts instead of rushing into things. Due to the cold weather, the O-ring had contracted and became too stiff to seal properly. This caused 7 innocent people to be killed.
This is a video showing the live recording of the Challenger explosion.
Thursday, February 26, 2009
Centre of Gravity
http://en.wikipedia.org/wiki/Center_of_mass
Sunday, February 22, 2009
The Upside Down World Map
Then, he showed me this "upside down worlds map" made in Australia. Australia is known as Down Under because it is, according to the world, near the bottom of the Earth. However, in this map, Australia is challenging this. This world map, as its name suggests, has Antarctica and Australia at the top of the world and South America on top of North America. It shows another way of looking at the Earth. Cool, isn't it?

This picture is kind of small, but you can click on it to view a page where it is much, much, much larger. Enjoy!
Thursday, February 12, 2009
The North Pole Issue
This is the site where I did my research. http://sg.answers.yahoo.com/question/index;_ylt=AkNOnOfUtpM8NsVKD_Nlolwh4wt.;_ylv=3?qid=20070726222930AAUa3Hd
Monday, February 9, 2009
Forces

Take this picture as an example. The four forces are acting on it with equal strength. Hence, the box does not move. If you look at FN and Fg, they are the two forces we are focusing on. The box is neither moving up nor down, hence the equal forces have no effect on it and the box is not being lifted.
Thursday, February 5, 2009
Quicksand
The same concept is applied with trying to get out of quicksand. You have to slowly and methodically move about towards the edge or something to grab onto. By keeping calm and not panicking, and taking deep breaths, you remain bouyant. Although it is a long and tedious process, you willl eventually be able to get out.
Wednesday, February 4, 2009
Meniscus 2 (Follow Up)
As for the other question... I suppose it would be very hard to even test it, as non-polar materials are likely to be opaque, and hence the result cannot be observed. However, if the theories are correct, liquids should not form a meniscus of any sort when they are placed in a container made of a non-polar material.
Tuesday, February 3, 2009
Meniscus

I researched on meniscus and found many answers, mostly too scientifc ones. However, I finally managed to come across a clear but simple explanation. There are two websites which I got my answer from.
Basically, water has polar molecues, just like glass. Polar molecuse tend to stick togther, so the water sticks to the edge of the measuring cylinder. Hence, the water will tend to form a curve and creep up the edge of the cylinder. This will result in the formation of a concave meniscus, as depicted in A. A convex meniscus, depicted in B, is very rare but may be formed by liquids like mercury where the molecules of the liquid have a stronger attraction to each other than to the container.
What I'm wondering is, if a cylinder of a different material is used, will it also form a meniscus and why? Surely not all material have polar molecues? And what does surface tension have to do with this?
These are the websites that I used for my research.
http://en.wikipedia.org/wiki/Meniscus
http://answers.yahoo.com/question/index?qid=20080917134724AArrlg8
Monday, February 2, 2009
Measurements and density
In my opinion, this question is no practical as no two substances are identical. Each substance is unique and hence no two substances have the same density. Even ice and water, different states of the same substance, have different densitieas ice is less dense than water. Hence, this is not likely to happen.
However, in the event that it does happen by a stroke of luck... If it were a liquid, both liquids would mix together and form a mixture. However, since it is a solid, perhaps it would be in the liquid itself as that is the closest it gets to mxing. Also, to float would mean it is less dense and to sink would mean it is denser, so it would probably be in between.
The second question is as follows: Water always has a meniscus at the suface where it curves instead of being fully straight. Why? Even Mr. Lim is not too sure but he says it has something to do with surface tension. I think it is because air is pressing against the surface of water, causing it to bend and curve slightly. I shall research more on this subject when I have time.
Friday, January 30, 2009
Scenario Presentation 2
This problem seems quite tricky. However, if you think hard about it, the solution is actually pretty simple.
The group had two solutions. 1. Use an electromagnet. Adjust the resistance of the electromagnet such that it attracts 50 screws each time. However, Mr. Lim said that this solution was not very relevant as the electromagnet would be likely to attract either all the screws or none at all. Hence, this solution was not likely to work.
2. Use a weighing scale. Count the first 50 screws and weigh them. Subsequently, grab a handful of screws and weigh them. Compare the reading to the first reading of 50 screw and adjust the number of screws accordingly. This solution is both practical and feasible. It is based on how shopkeepers weigh biscuits or ham using the weighing scale and add more or take out some based on the weight of the products.
The next group had a relatively more common scenario, but explaining it was more difficult. This was their problem: A spring balance will give a different reading on the Moon than from that on Earth. A beam balance, however, will give the same reading. Explain why.
The group's answer is as follows: A spring balance measures weight, which is dependant on gravity. The Moon has less gravity than the Earth, hence the reading differs. However, a beam balance measures mass, which is constant no matter what. Short and sweet, but very clear.
The final group had a very interesting topic. How do submarines float and sink at will?
In my opinion, this group had the most detailed presentation. They explained how air is pumped into the ballast tanks to force water out so that the submarine would float. On top of that, they included a lot of other information such as diagrams, and information on the Kursk Russian submarine disaster. They talked about how a faulty Dummy torpedo had allowed flammable liquid to leak through and explode the front of the submarine, letting water in and causing the submarine to sink.
At the end of the presentation, they gave us question to think about: If a submarine sinks to the seabed, why is it no longer available to move off? The answer is that there is no water under the submarine to create a lift force, hence the submarine is stuck there until rescue workers drop a rope down to haul it back up. I enjoyed this group's presentation the most.
I enjoy thought-provoking lessons and topics like the ones Mr. Lim gave us. I hope we'll be able to have more of such lessons in the future.
Wednesday, January 28, 2009
Scenario Presentation Part 1
They certainly thought through their scenario very well. The group gave two possible solutions. The first was to use the scale to calcuate the length and width of the reservoir, then multiply it by the depth for the final answer. The second was to draw the shape of a cylinder on the map. The cylider covered most of the reservoir. After that, they used a formula to calculate the volume of the cylinder and used the scale to convert it to live size. I found the presentation clear, concise, well-thought and interesting.
It was our turn next. I think we did okay. At least no one seemed confused when we explained our method.
We were supposed to finish off all the group presentations, but we were running late again.
Scenario solving
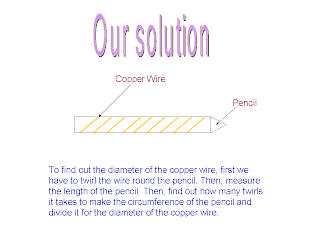
You are given a length of copper wire with a small diameter. The only measuring instrument available to you is the meter ruler. Explain how you would measure the diameter of the wire with an acceptable degree of accuracy.
At first, we were stumped as it seemed impossible. Then, Mr. Lim told us we could have a pencil to use as part of the materials. After that, everything just snapped into place. We came up with a simple but effective solution:
To find out the diameter of the copper wire, first we have to twirl the wire round the pencil. Then, measure the length of the pencil. Then, find out how many twirls it takes to make the circumference of the pencil and divide it for the diameter of the copper wire.
Pretty neat, isn't it?